The state of water vapour
Water can exist in a liquid, gas (water vapour) or solid (ice) state in the atmosphere, with individual molecules continuously evaporating (going from the liquid to gas state), condensing (going from the gas to liquid state), subliming (going from the solid to the gas state) and depositing (going from the gas to the solid state).
But what governs which of those processes occurs where and when?
Basically, they are all occurring all the time, it is just the rate at which they are occurring that varies.
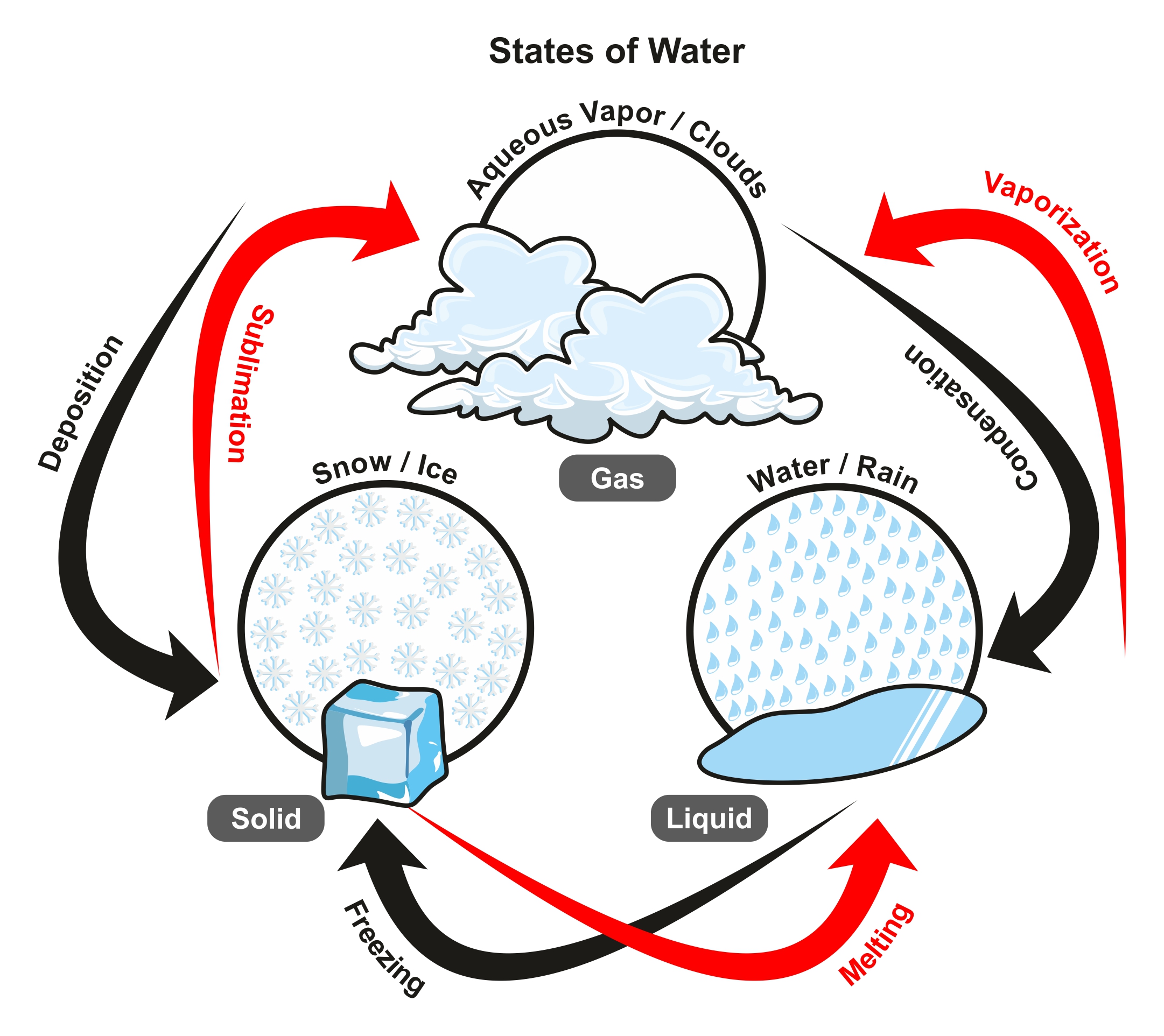
Evaporation, for example, mostly depends on temperature — the warmer it is, the faster the rate of evaporation from lakes, rivers, puddles, vegetation, our skin, and cloud droplets.
Condensation, on the other hand, mostly depends on the concentration of water vapour already in the air. The more humid the air, the fast the rate of condensation.
If there is more evaporation going on than condensation, the sky is clear. If there is more condensation occurring than evaporation, cloud droplets form — the temperature at which this happens is called the dew point temperature. At the dew point temperature, the relative humidity of the air is 100% and it is said to be saturated.
So, what do we mean by ‘relative humidity’? Relative humidity is the amount of water vapour present in air expressed as a percentage of the amount needed to achieve saturation at the same temperature.
Cloud can form either when the amount of water vapour in the air increases without changing the temperature of the air — this increases the rate of condensation, or by cooling the air down without changing the amount of water vapour in the air — this decreases the rate of evaporation.

Have you ever watched a puffy cumulus cloud on a warm, sunny day? You can literally see parts of the cloud evaporating whilst other parts grow. Have you also noticed that, unlike most primary school drawings, cumulus clouds have flat bases? Cloud base marks the level in the atmosphere which is at the dew point temperature — below cloud base there is more evaporation going on than condensation, above cloud base there is more condensation.
Have you ever heard the expression ‘it’s too cold for snow’? What it really means is ‘it’s too dry for snow’ — in the centre of large continents such as N America and Antarctica, in the very cold winter, where you are a long way away from large bodies of liquid water, the rate of sublimation or evaporation is so low that the air is very, very dry and no snowflakes can form.
It’s also worth noting that, when we talk about evaporation rates, it’s the rate at which water would evaporate from the flat surface of a lake that’s meant. In reality, it’s much easier for a water molecule to escape from a curved surface — the more curved the surface, the fewer nearby molecules holding it the water molecule place. So, the smaller a raindrop, the easier it is for it to evaporate.
In practice, this means that cloud droplets tend to form on a small particle — soot, salt, pollen, even bacteria — this makes them instantly bigger and therefore makes it harder for water molecules to escape. At times, there aren’t enough of these small particles, known as Cloud Condensation Nuclei, in the air — and, if this is the case, the relative humidity can be over 100%; it is super-saturated. This is one example of why defining saturation (100% relative humidity) as ‘the amount of water vapour the air can hold’ is really unhelpful.